The Innovation Fellows Program concentrates on developing medical devices for a broad variety of clinical areas. The goal is to train the next leaders in medtech by fostering leadership and teaching risk management for medical devices. Innovation Fellows learn a disciplined medical technology product development process which includes FDA requirements, reimbursement, intellectual property and business strategies in addition to creativity techniques and prototyping.
Accomplishments
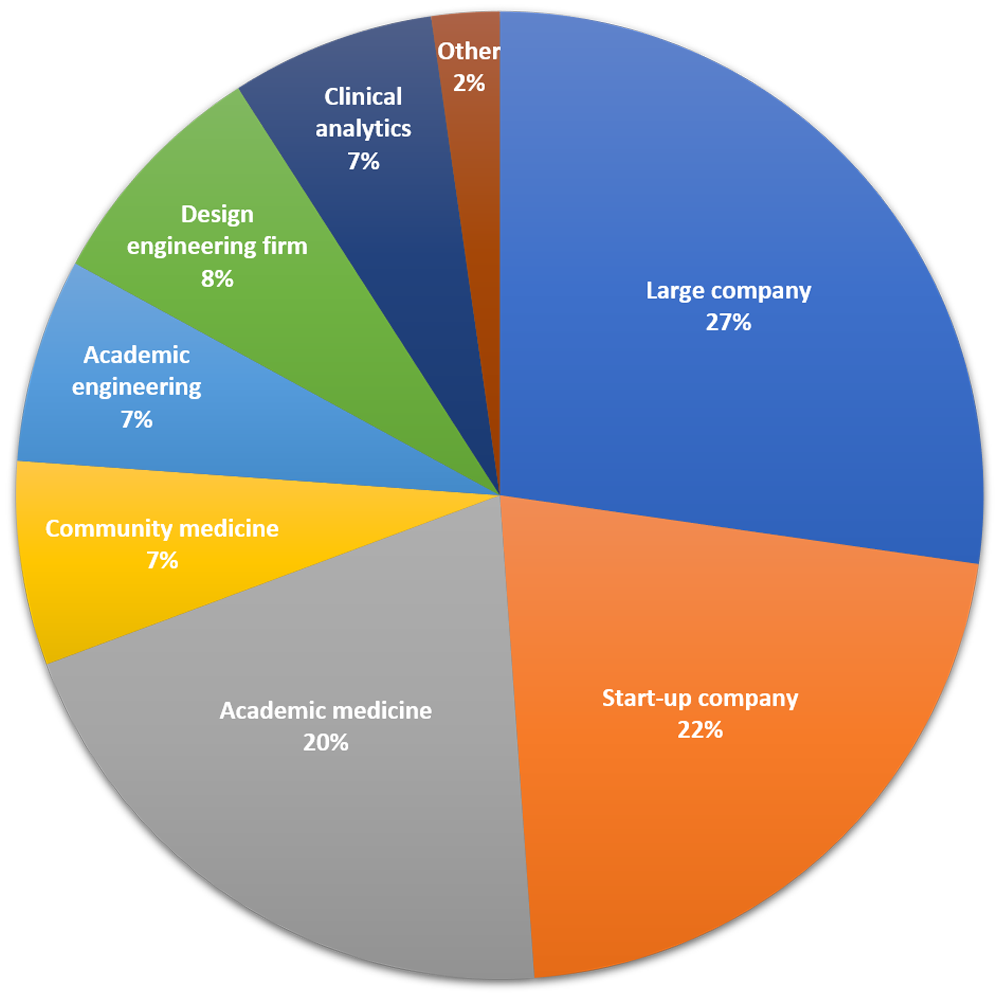
The Innovation Fellows Program is an intense, immersion experience that identifies current medical device needs and innovates solutions. The Fellows translate ideas into inventions, develop prototypes, and conduct preliminary stages of testing.
Since the program’s inception in 2008, there have been:
- 297 Invention Disclosures
- 147 Patent Applications
- 160 Technology Licenses Executed
- 16 Patents Issued
- 77 Alumni Fellows
- 16 Start-up Companies
- 34 Products Licensed/Optioned/Donated
- over $35M Funding Raised by former Innovation Fellows
Become a Fellow
Be an Innovator!
Thank you for your interest in the University of Minnesota Bakken Medical Devices Center Innovation Fellows Program.
No Longer Accepting Applications for 2023-2024 Team.
Current Fellows

Suhas Bajgur
Interventional Neuro-Radiologist

Nathan Johnson
Manufacturing Engineer

Joseph Broomhead
3D Bioprinting, Vascular Surgery

Shahid Karim
Cardiac/Respiratory Physiology, UK Interventional Cardiologist

Qiman Gao
Facial Pain Fellow, Dentist

Danny Sachs
Program Director